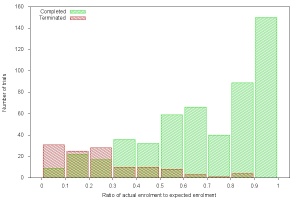
Ratio of actual enrolment to expected enrolment versus number of trials for trials that completed and trials that terminated due to poor accrual in 2011
The moral acceptability of a clinical trial is rooted in the risk and benefit for patients, as well as the ability of the trial to produce generalisable and useful scientific knowledge. The ability of a clinical trial to justify its claims to producing new knowledge depends in part on its ability to recruit patients to participate—the fewer the patients, the less confident we can be in the knowledge produced. So when trials have recruitment problems, those trials also have ethical problems.
In a recently published issue of Clinical Trials, my colleagues and I investigate the prevalence of poor trial accrual, the impact of accrual problems on study validity and their ethical implications.
We used the National Library of Medicine clinical trial registry to capture all initiated phase 2 and 3 intervention clinical trials that were registered as closed in 2011. We then determined the number that had been terminated due to unsuccessful accrual and the number that had closed after less than 85% of the target number of human subjects had been enrolled.
Of 2579 eligible trials, 481 (19%) either terminated for failed accrual or completed with less than 85% expected enrolment, seriously compromising their statistical power. A total of 48,027 patients had enrolled in trials closed in 2011 who were unable to answer the primary research question meaningfully.
Not only that, but we found that many trials that should have been terminated were pursued to completion, despite flagging rates of subject accrual, and the proportion of trials that completed was much higher than the proportion of trials that terminated, even at accrual levels as low as 30%. (See attached figure.)
The take-home message is that ethics bodies, investigators, and data monitoring committees should carefully scrutinize trial design, recruitment plans, and feasibility of achieving accrual targets when designing and reviewing trials, monitor accrual once initiated, and take corrective action when accrual is lagging.
BibTeX
@Manual{stream2014-615,
title = {Unsuccessful trial accrual and human subjects protections: An empirical analysis of recently closed trials},
journal = {STREAM research},
author = {Benjamin Gregory Carlisle},
address = {Montreal, Canada},
date = 2014,
month = nov,
day = 6,
url = {http://www.translationalethics.com/2014/11/06/trial-accrual-and-ethics/}
}
MLA
Benjamin Gregory Carlisle. "Unsuccessful trial accrual and human subjects protections: An empirical analysis of recently closed trials" Web blog post. STREAM research. 06 Nov 2014. Web. 26 Apr 2025. <http://www.translationalethics.com/2014/11/06/trial-accrual-and-ethics/>
APA
Benjamin Gregory Carlisle. (2014, Nov 06). Unsuccessful trial accrual and human subjects protections: An empirical analysis of recently closed trials [Web log post]. Retrieved from http://www.translationalethics.com/2014/11/06/trial-accrual-and-ethics/

Leave a Reply